In the world of high-performance nickel alloys, choosing between Hastelloy B2 (UNS N10665) and Hastelloy C276 (UNS N10276) can be the difference between a decade of service and a catastrophic equipment failure. While both belong to the Hastelloy family, their chemical DNA dictates vastly different responses to corrosive media. This guide breaks down the technical nuances to help engineers make an informed decision.
B2 vs C276 comparison
The fundamental difference between these two alloys lies in their Хром (Cr) content. Hastelloy B2 is essentially a nickel-molybdenum alloy with almost no chromium, whereas C276 is a nickel-molybdenum-chromium alloy with the addition of tungsten.
| Элемент | Hastelloy B2 (Typical %) | Hastelloy C276 (Typical %) |
| Никель (Ni) | Balance (approx. 68%) | Balance (approx. 57%) |
| Молибден (Mo) | 26.0 – 30.0 | 15.0 – 17.0 |
| Хром (Cr) | 1.0 макс. | 14.5 – 16.5 |
| Железо (Fe) | 2.0 макс. | 4.0 – 7.0 |
| Вольфрам (W) | – | 3.0 – 4.5 |
| Кобальт (Co) | 1.0 макс. | 2,5 макс. |
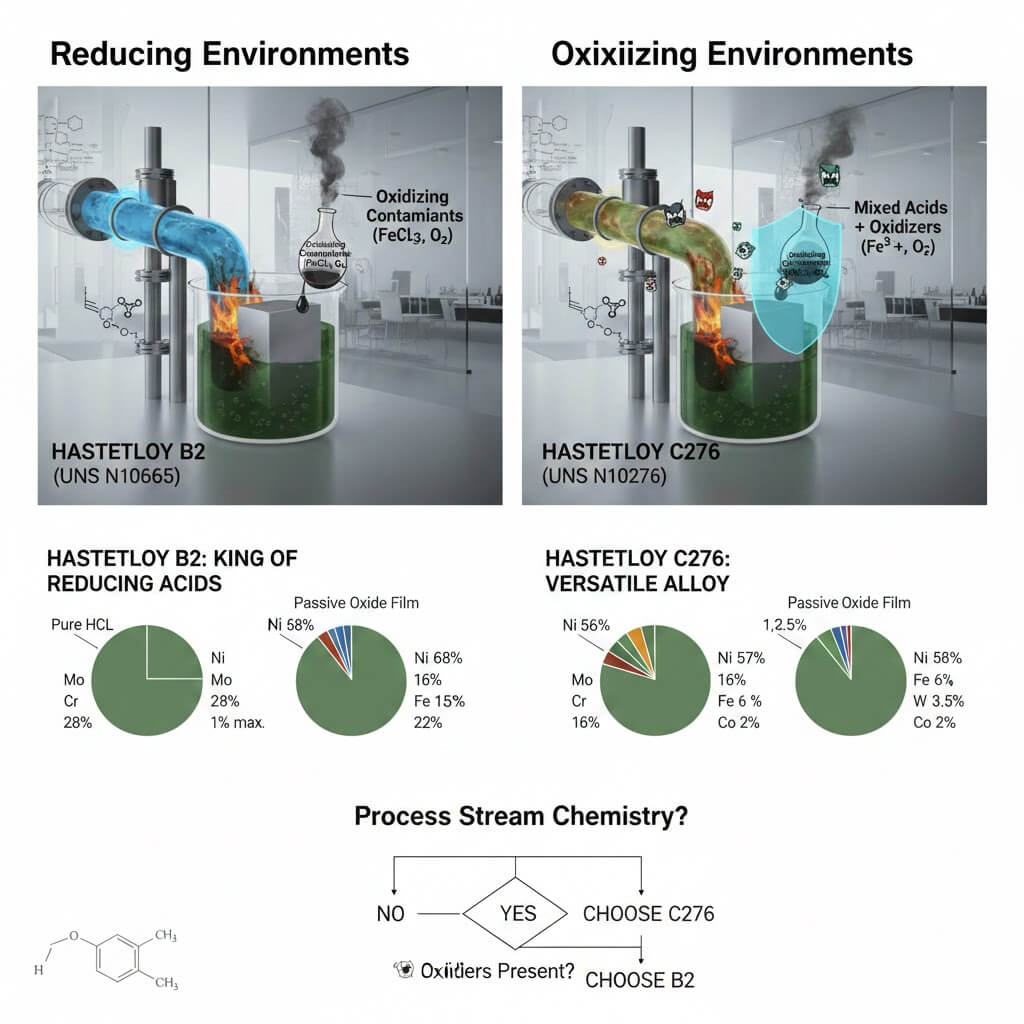
Hastelloy B2 is designed for extreme resistance to hydrochloric acid. Its high molybdenum content provides structural integrity in high-temperature reducing environments. However, the lack of chromium makes it vulnerable to even trace amounts of oxidizing contaminants.
Хастеллой C276 is widely considered the “versatile” alloy. The 16% chromium content creates a passive oxide film that protects the metal from oxidizing salts and gases, while the molybdenum and tungsten provide exceptional resistance to pitting and crevice corrosion in chloride-rich environments.
Hastelloy B2 vs C276 reducing vs oxidizing environments
Understanding the chemistry of your process stream is vital when comparing B2 vs C276.
-
Reducing Environments: Hastelloy B2 is the undisputed king here, particularly in all concentrations of Hydrochloric Acid (HCl) at temperatures up to the boiling point. It also performs exceptionally well in pure sulfuric acid and phosphoric acid under reducing conditions. Because it lacks chromium, it does not form an oxidizing protective layer, allowing the molybdenum to work directly against the reducing acid ions.
-
Oxidizing Environments: This is where Hastelloy B2 fails and C276 shines. If your system contains Ferric ions, Cupric ions, or dissolved Oxygen, B2 will undergo rapid, catastrophic corrosion. Hastelloy C276, thanks to its chromium content, can handle “mixed” acids—streams that are primarily reducing but contain oxidizing impurities. It is the go-to material for flue gas desulfurization (FGD) and complex chemical processing where the environment might fluctuate.
B2 vs C276 how to choose
Choosing the right grade requires a deep dive into the specific contaminants of your media. Follow these three engineering rules:
-
Check for Oxidizers: If there is any chance of oxygen, chlorine gas, or oxidizing salts (like FeCl3) being present, choose C276. B2 is strictly for pure reducing acids.
-
Evaluate HCl Concentration: For high-concentration, high-temperature pure HCl where C276 might show moderate corrosion rates, B2 is the superior technical choice.
-
Stress Corrosion Cracking (SCC) & Pitting: If the application involves stagnant “dead zones” where chlorides can concentrate, the molybdenum and tungsten in C276 offer better protection against localized pitting than B2.
Связанные вопросы и ответы
Q1: Can Hastelloy B2 be used in nitric acid applications?
No. Nitric acid is a strong oxidizing agent. Hastelloy B2 lacks the chromium necessary to form a protective passive film and will corrode almost instantly. Hastelloy C276 or even C22 would be much more appropriate.
Q2: Why is Hastelloy B3 often mentioned alongside B2?
Hastelloy B3 is the modern successor to B2. It offers the same corrosion resistance but has improved thermal stability, making it easier to fabricate and less prone to embrittlement during welding or heat treatment.
Q3: Which alloy is better for “Sour Gas” (H2S) environments?
Hastelloy C276 is the industry standard for sour gas service in the oil and gas sector. Its resistance to sulfide stress cracking (SSC) and pitting in the presence of chlorides and H2S makes it superior to B2 for these complex, often slightly oxidizing, environments.